Fill in a Valid 96 Well Template
The 96 Well form is a standardized document used primarily in laboratory settings to organize and record data from experiments conducted in 96-well plates. This form facilitates efficient data collection and analysis, ensuring that researchers can easily track results across multiple samples. Its structured format helps maintain clarity and accuracy in scientific research.
Get Document Online
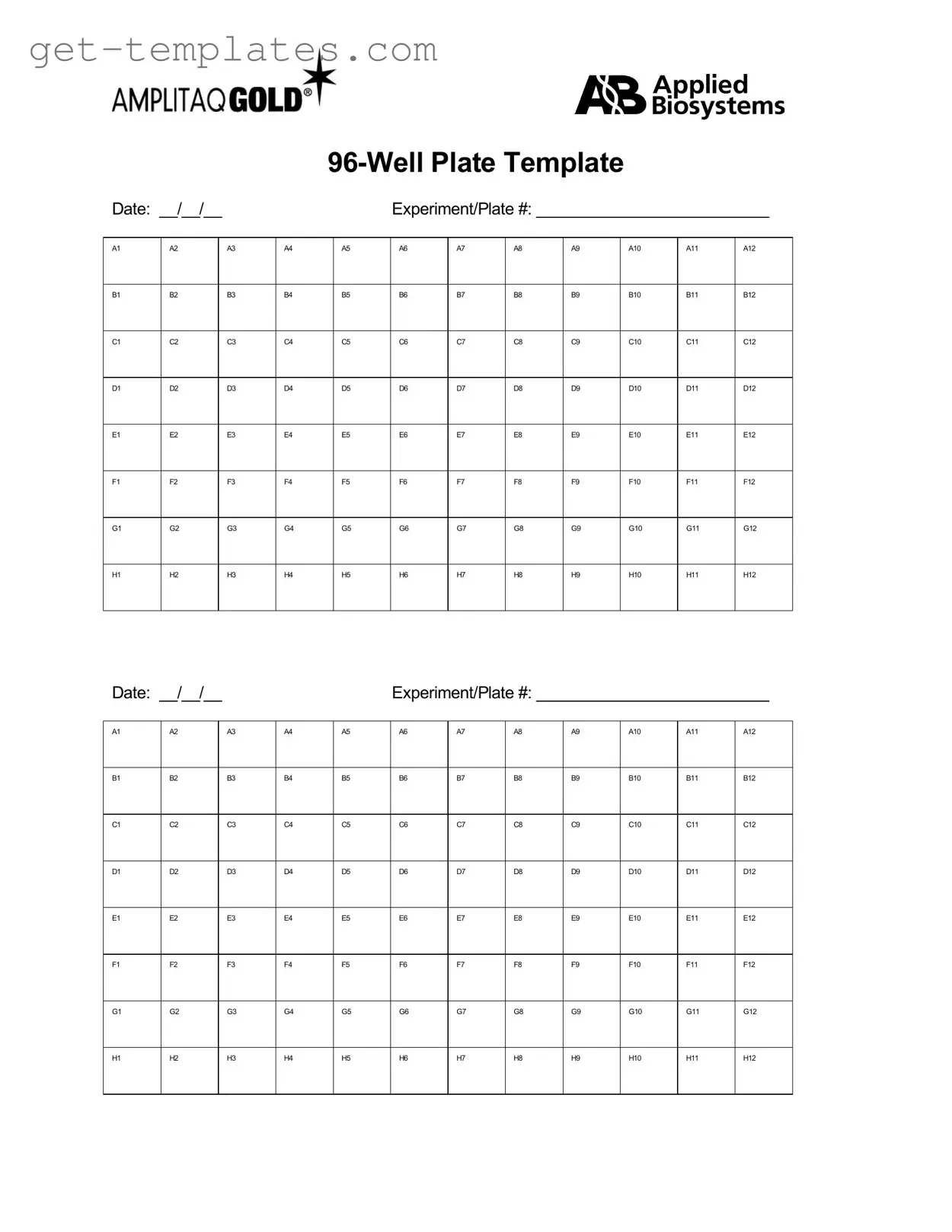
Fill in a Valid 96 Well Template
Get Document Online
You’re halfway through — finish the form
Finish 96 Well online — edit, save, download made easy.
Get Document Online
or
⇓ PDF Form

